Atmospheric Pressure
Atmospheric Pressure: Overview
This topic covers concepts, such as, Atmospheric Pressure, Variation of Atmospheric Pressure with Height & Height of Atmosphere etc.
Important Questions on Atmospheric Pressure
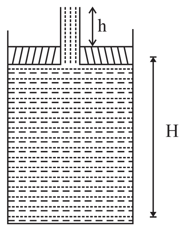
A piston of mass M = 3kg and radius R = 4cm has a hole into which a thin pipe of radius r = 1cm is inserted. The piston can enter a cylinder tightly and without friction, and initially it is at the bottom of the cylinder. 750gm of water is now poured into the pipe so that the piston & pipe are lifted up as shown. Find the height H of water in the cylinder h of water in the pipe
The atmospheric pressure at a surface depends on the height of the air column above it.
What is the cause of the pressure of atmosphere?
Why the passengers are advised to remove the ink from their pens while going up in an aeroplane?
Assuming that the atmosphere has the same density anywhere as at sea level and to be constant . What should be the approximate height of the atmosphere
We know that at high altitudes the partial pressure of oxygen is less than that at the ground level. Now what are the consequences of this
A tank with a square base of area is divided into two compartments by a vertical partition in the middle. There is a small hinged door of face area at the bottom of the partition. Water is filled in one compartment and an acid of density in the other, both to a height of . The horizontal force necessary to keep the door closed is (Take
In equation number , if of water and spirit each are further poured into the respective arms of the tube, difference in the level of mercury in the two arms is (Take, relative density of mercury= )
A tank is filled with a liquid upto a height H. A small hole is made at the bottom of this tank. Consider be the time taken to empty first half of the tank and be the time taken to empty rest half of the tank. Then, determine the ratio
A narrow glass tube, 80 cm long and opens at both ends, is half immersed in mercury, now the top of the tube is closed and is taken out of mercury. A column of mercury 20 cm long remains in the tube. Find atmospheric pressure
Calculate the difference in water levels in two communicating capillary tubes of radius and .
(Surface tension of water is )
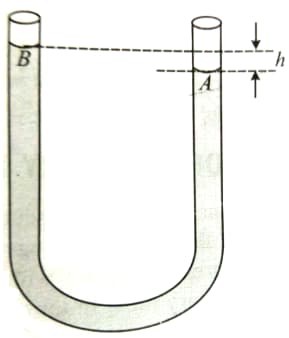
If the container pictured below is filled with an ideal fluid, which point in the fluid most likely has the greatest pressure-
An open ended manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Atmospheric pressure is of . What is the pressure of the trapped gas-
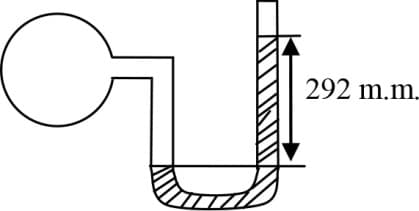
A vertical tube of length contains a mercury pallet of length as shown in figure. The length of tube above mercury pallet if the tube is inverted is nearly :
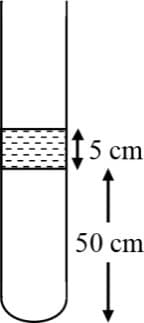
A incompressible and non-viscous liquid of density is kept in a cylindrical container having a opening at . Then,
The pressure on a swimmer 20 m below the surface of water at sea level is
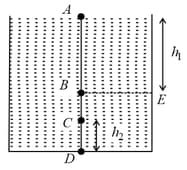
The top surface of an incompressible liquid is open to the atmosphere. The pressure at a depth How does the pressure at depth compare with
A glass tube long and open at both ends is half immersed in mercury. Then the top of the tube is closed and it is taken out of the mercury. A column of mercury long then remains in the tube. The atmospheric pressure (in of ) is
The top surface of an incompressible liquid is open to the atmosphere. The pressure at a depth How does the pressure at depth compare with
